Roots Analysis has announced the addition of “Clinical Trials Software Market, 2022-2035” report to its list of offerings.
Excessive capital expenditure and other complexities associated with the traditional clinical trials has imposed an enormous financial burden on the pharmaceutical industry. Virtual clinical trials software solutions have the potential to induce substantial digital changes in clinical research methodology, resulting in a more patient-centric, cost-effective and easy to manage approach.
Key Market Insights
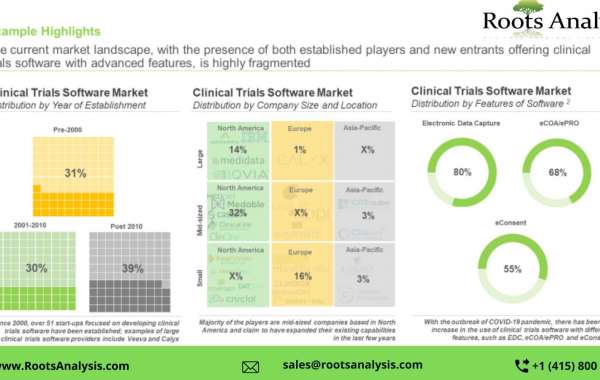
Over 70+ companies claim to provide clinical trial software
The companies offer clinical trials software with different features like electronic data capture, eCOA/ePRO and eConsent along with decentralized and virtual clinical trials and remote monitoring of the patients. Majority of the players based in North America offer clinical trials software followed by Europe and Asia-Pacific. Further, the market is dominated by the presence of mid-sized players (more than 40%) followed by small and large players.
Clinical trial management software is a well-known fact that clinical trials form an integral part of the overall drug development process, enabling innovators to assess safety and efficacy of their drug candidates / devices. These studies account for around 50% of the total time and capital invested in the development process
Since 2016, more than 120+ partnerships have been inked by service providers
Interestingly, the maximum number of partnership agreements were inked in 2021, majority of these were service agreements (44%), followed by acquisitions/mergers (26%). Further, most of the deals were inked with players based in North America (64%).
Over 30 mergers and acquisitions were reported in this domain, during the period 2016-2021
More than 85% of these were instances of acquisitions. Further, majority of the instances involved the companies based in North America and the maximum number of deals were inked in 2019.
Over USD 492.8 million has been invested by both private and public investors, since 2016
Majority of the companies (67%) engaged in this domain primarily received funding through venture capital rounds. Further, around 98% of the funding instances were reported by players headquartered in North America.
The market is expected to grow at an annual rate close to 14% over the coming decade
The opportunity is likely to be well distributed across clinical trials software on the basis of features of software (EDC, eCOA/ePRO and eConsent) and geographies (North America, Europe and Asia-Pacific). By 2035, the clinical trials software market in North America is anticipated to grow at a relatively faster pace (39%), followed by the market in Europe (21%).
Clinical trial management system: Driven by the substantial progress in this domain, encouraging virtual clinical trial results, and ongoing technological advancement, the clinical trials software market is anticipated to grow at a commendable pace in the mid to long term.
To request a sample copy / brochure of this report, please visit https://www.rootsanalysis.com/reports/clinical-trial-software-market/request-sample.html
Key Questions Answered
- Who are the leading players engaged in the development of clinical trials software solutions?
- Which region(s) will occupy the maximum market share in clinical trials software domain?
- Who are the key venture capitalists / strategic investors funding the clinical trials software development initiatives?
- Which partnership models are commonly adopted by stakeholders engaged in the development of clinical trials software solutions?
- Which factors are likely to influence the evolution of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
By 2035, the financial opportunity within the clinical trials software market has been analysed across the following segments:
- Features of Software
- Electronic Data Capture
- eCOA/ePRO
- eConsent
- Analysis by Geographical Regions
- North America
- Europe
- Asia Pacific
The research includes profiles of key players (listed below); each profile features a tabulated overview of company, product portfolio, recent developments, and an informed future outlook.
- Advarra
- ArisGlobal
- AssistRx
- Clario
- IBM
- IQVIA
For additional details, please visit
https://www.rootsanalysis.com/reports/clinical-trial-software-market.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
- Flow Cytometry Service Market, 2022-2035
- Gene Editing beyond CRISPR Market, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091