Key Inclusions
- A general overview of mRNA, highlighting details on its structure and the historical evolution of mRNA vaccines. It also presents information on the in-vitro synthesis of mRNA, its applications in various therapeutic modalities and the challenges associated with the process. Additionally, it features a discussion on the commonly outsourced manufacturing operations and their
- A detailed assessment of the overall market landscape of the companies offering mRNA custom synthesis services, based on several relevant parameters, such as year of establishment, company size (in terms of number of employees), location of headquarters, type of service(s) offered (custom synthesis, modification, purification, process development, scale-up / manufacturing and fill / finish), input for synthesis (plasmid DNA, mRNA sequence, PCR fragments and other(s)), structural modification (3’ modification, 5’ modification and base modification), type of purification method(s) (precipitation, chromatography, electrophoresis and other(s)), application area(s) (research and therapeutics / vaccines), scale of operation (research / preclinical, clinical, and commercial) and GMP compliance. It also features information on the mRNA synthesis / manufacturing capacity of the mRNA custom synthesis service providers, highlighting the length of the mRNA manufactured.
- A detailed competitiveness analysis of mRNA custom synthesis service providers based on supplier strength (in terms of years of experience) and service strength (considering type of service(s) offered, type of modification, scale of operation, GMP compliance and application areas).
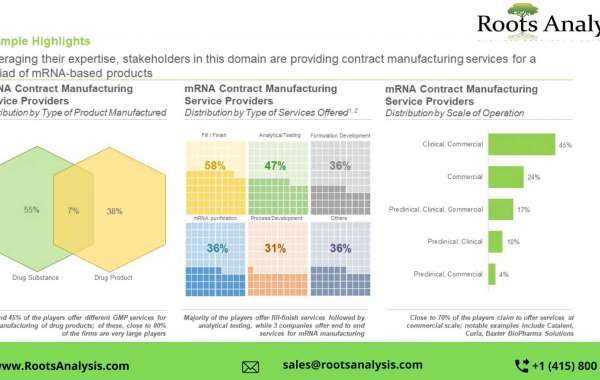
- A comprehensive assessment of the overall market landscape of mRNA contract manufacturing service providers, based on several relevant parameters, including year of establishment, company size (in terms of number of employees), location of headquarters, location of manufacturing facilities, type of product(s) manufactured (drug products (FDFs) and drug substances (APIs)), type of service(s) offered (fill / finish, formulation development, purification, process development, analytical testing, and regulatory support) and scale of operation (research / preclinical, clinical and commercial).
- An in-depth company competitiveness analysis of mRNA contract manufacturing service providers based in North America, Europe and Asia-Pacific. The analysis compares the contract service providers based on supply strength (in terms of years of experience) and service portfolio strength (considering type of service(s) offered, scale of operation, type of product manufactured and number of facilities).
- A detailed overview of the current market landscape of kits available for mRNA synthesis, based on several relevant parameters, such as type of enzyme, kit components, type of enzyme mix used, mRNA component modified, yield per reaction, number of reactions, reaction run time and kit price. It also features a list of players engaged in the development of mRNA kits, along with analysis based on year of establishment, company size (in terms of number of employees) and location of headquarters. Additionally, it highlights the leading players (in terms of number of mRNA synthesis kits offered) in this domain.
- A product competitiveness analysis of the mRNA synthesis kits, based on supplier power (in terms of the experience of the developer) and product competitiveness (in terms of type of enzyme, number of kit component(s), type of mRNA component modified, yield, number of reaction(s) and price).
- Elaborate profiles of key players engaged in the synthesis and manufacturing of mRNA (shortlisted based on strength of service portfolio). Each profile includes a brief overview of the company, along with information on mRNA synthesis service portfolio, additional services offered, recent developments and an informed future outlook.
- Detailed profiles of the key mRNA synthesis kit providers (shortlisted based on strength of product portfolio). Each profile includes a brief overview of the company, along with information on the mRNA synthesis kits portfolio.
- An analysis of completed, ongoing and planned clinical studies of mRNA therapeutics and vaccines, based on several relevant parameters, such as trial registration year, trial status, trial phase, target patient population, therapeutic area, type of sponsor / collaborator, leading industry players (in terms of number of trials conducted), and regional distribution of trials.
- A detailed analysis of the recent partnerships inked between stakeholders engaged in this domain, since 2014, covering acquisitions, manufacturing agreements, fill / finish service agreements, supply agreements, service alliance, and other relevant deals.
- An in-depth analysis of over 35 mRNA-based therapeutics / vaccines developers that are likely to partner with mRNA contract manufacturing service providers. These players have been shortlisted based on several relevant parameters, such as developer strength (which takes into account the company’s experience), company size, (in terms of number of employees), pipeline strength (based on the number of mRNA-based drugs in pipeline), highest phase of development, therapeutic area, route of administration and type of candidate being developed.
- A review of the various mRNA-focused initiatives undertaken by big pharma players (shortlisted on the basis of the revenues generated in 2021), featuring various insightful representations, such as Harvey ball analysis, spider web analysis, based on several relevant parameters such as funding amount raised, partnership activity, and diversity of product portfolio (in terms of disease indication(s) being treated and focus therapeutic area(s)).
- A detailed market forecast, featuring analysis of the current and projected future opportunity across key market segments (listed below)
- Type of Product
- Drug substances (APIs)
- Drug products (FDFs)
- Application Area
- mRNA-based vaccines
- mRNA-based therapeutics
- Therapeutic Area
- Infectious diseases
- Oncological disorders
- Other diseases
- Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and North Africa
- Rest of the World
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/mrna-synthesis-and-manufacturing-market.html
You may also be interested in the following titles:
Decentralized Clinical Trials / Virtual Clinical Trials Market |
You may also like to learn what our experts are sharing in Roots educational series:
Cell Therapy – The Revolutionary Therapeutic Modality: All Set To Obliterate Oncological Disorders |
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415