Currently, more than 285 gene therapies are being evaluated in different phases of clinical development. Further, various gene therapy developers have raised more than USD 10 billion capital during the year 2021. With the growing interest in such therapies, the demand for novel delivery vectors has also increased. Among various gene delivery vectors available, adeno-associated viral vectors have emerged as one of the most efficient viral vectors. Till now, the USFDA has approved two adeno-associated viral vectors-based drugs, LUXTURNA® and ZOLGENSMA®.
In order to cater to the demand, close to 100 players, across the globe, have emerged for the development and manufacturing of adeno-associated viral vectors. In fact, a number of these companies also offer advanced technology platforms, enabling the processing of AAV vectors and related therapies across different scales of operation. Various industry and non-industry players are actively engaged in research and development of novel gene delivery technologies, which are safe and effective.
155+ Clinical Trials have been / are being conducted
The stakeholders have invested extensively in conducting clinical trials for evaluating the efficacy of the therapeutic candidates in the treatment of a variety of indications.
~4,300 Patent Filed / Granted in the Domain
Examples of leading industry stakeholders, in terms of most patent applications filed include Genzyme, Voyager Therapeutics, REGENXBIO, Baxalta and Spark Therapeutics.
200+ Partnerships and Collaborations signed in the Domain
The growing partnerships and collaborations signed between different stakeholders is indicative of the significant demand of vectors and related therapies from the past few years.
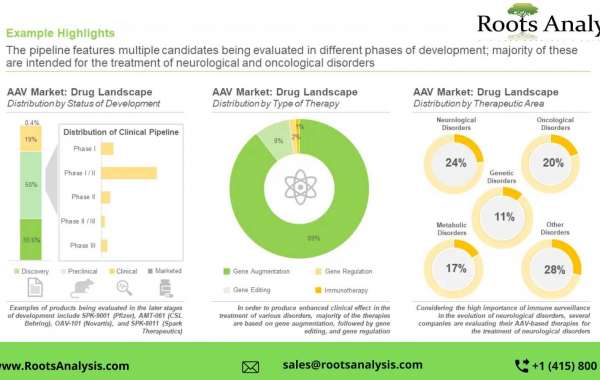
As projected by Roots Analysis, oncological disorders therapeutic area will have majority share in the adeno-associated viral vector market. As projected by Roots Analysis, the overall market of AAV vector manufacturers market is expected to expand in the coming years.
For additional details, please visit https://www.rootsanalysis.com/blog/adeno-associated-viral-vector/
You may also be interested in the following titles:
- Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
- AI-based Digital Pathology / AI Pathology Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com